Life Sciences Manufacturing ERP Software

A Complete Overview of BatchMaster Manufacturing
Software for Life Sciences and Nutra
OUR LIFE SCIENCES SOFTWARE SOLUTIONS HELPS YOU IMPROVE OUR HEALTH AND LENGTHEN OUR LIVES
BatchMaster Software offers life sciences manufacturing applications to formulate and produce enzymes and biological substances, as well as pharmaceutical products, such as generic, OTC and prescription drugs. Our life sciences and pharmaceutical software solutions will help your company streamline operations and bring your products to market, faster and more cost efficiently.
From a financial and liability perspective, our pharmaceutical ERP supports organizations that are organized into multiple divisions or business entities, ranging from sales to marketing to manufacturer to distributor, where manufacturing and distribution may be managed by one or more outside contract manufacturers. Each business partner can manage their own process requirements (e.g. product development, production), and has the means to easily transfer ownership of products and share data amongst partners.
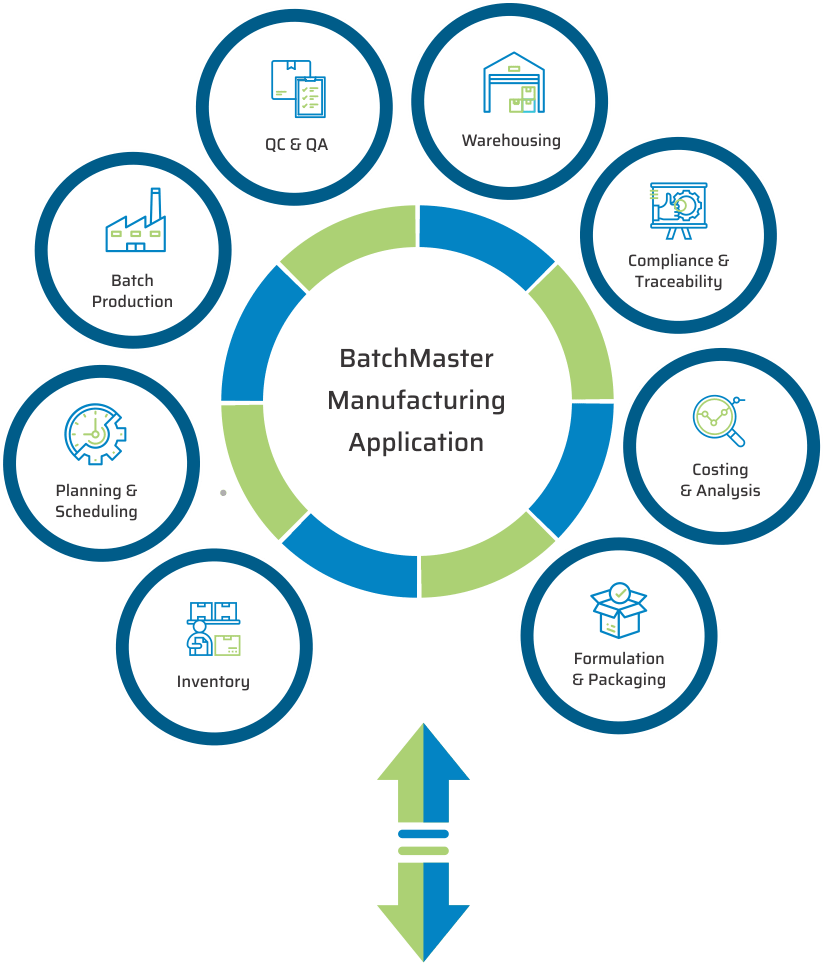
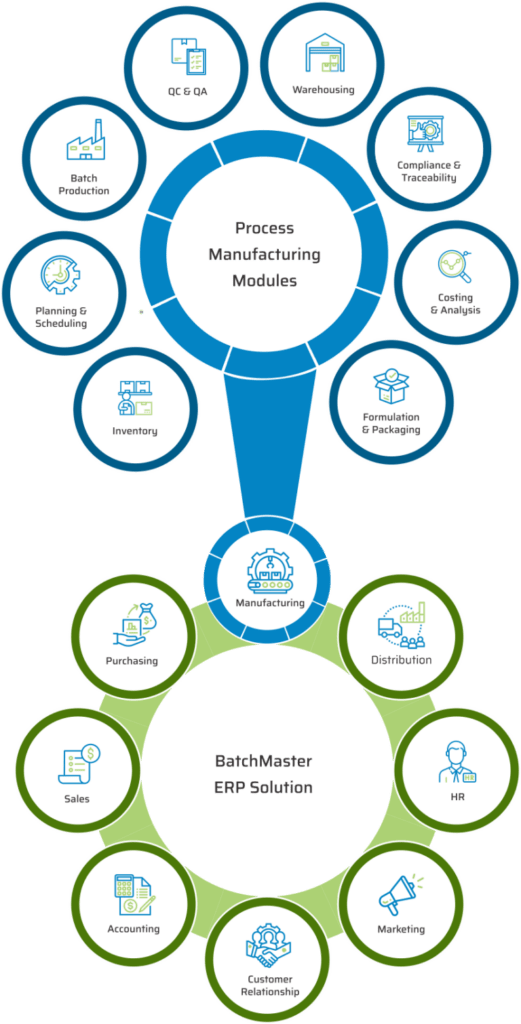
KEY MODULES FOUND IN OUR LIFE SCIENCES & PHARMACEUTICAL MANUFACTURING APPLICATION
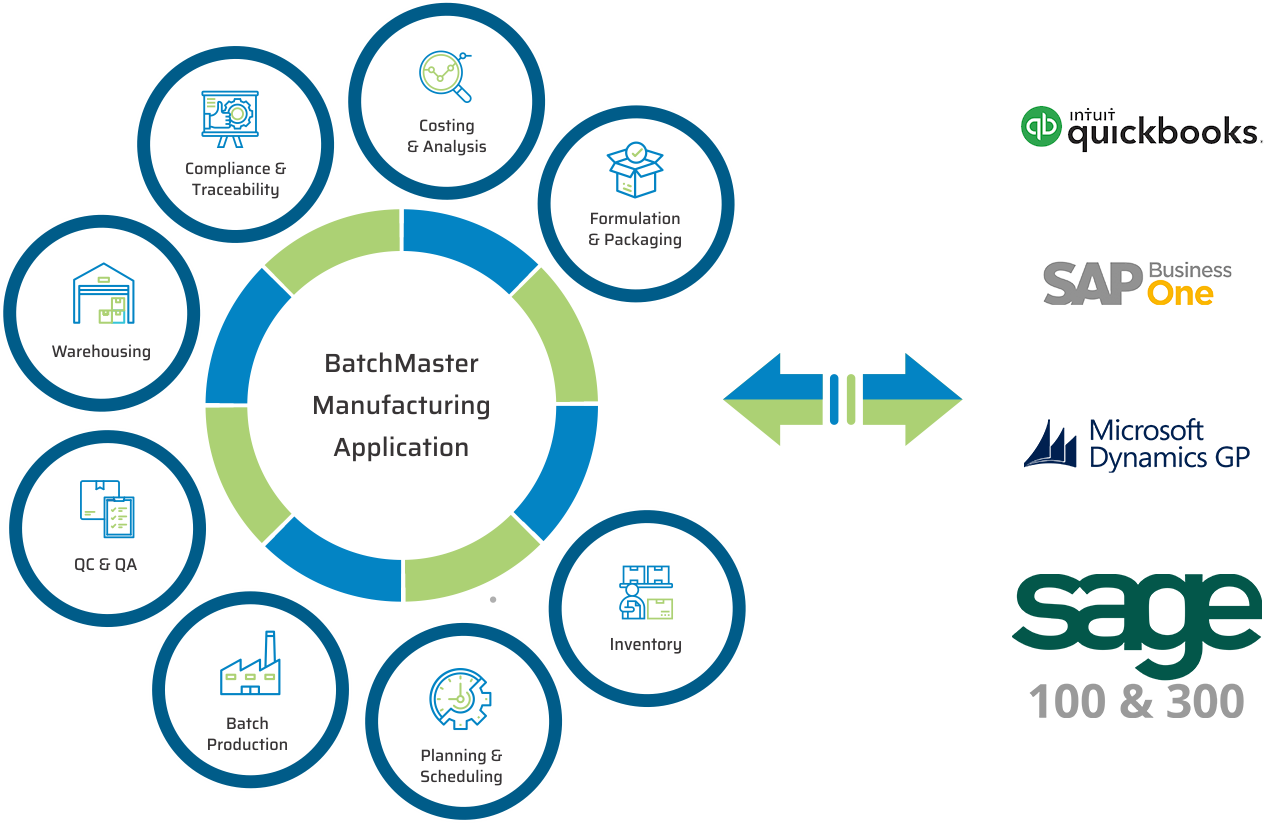
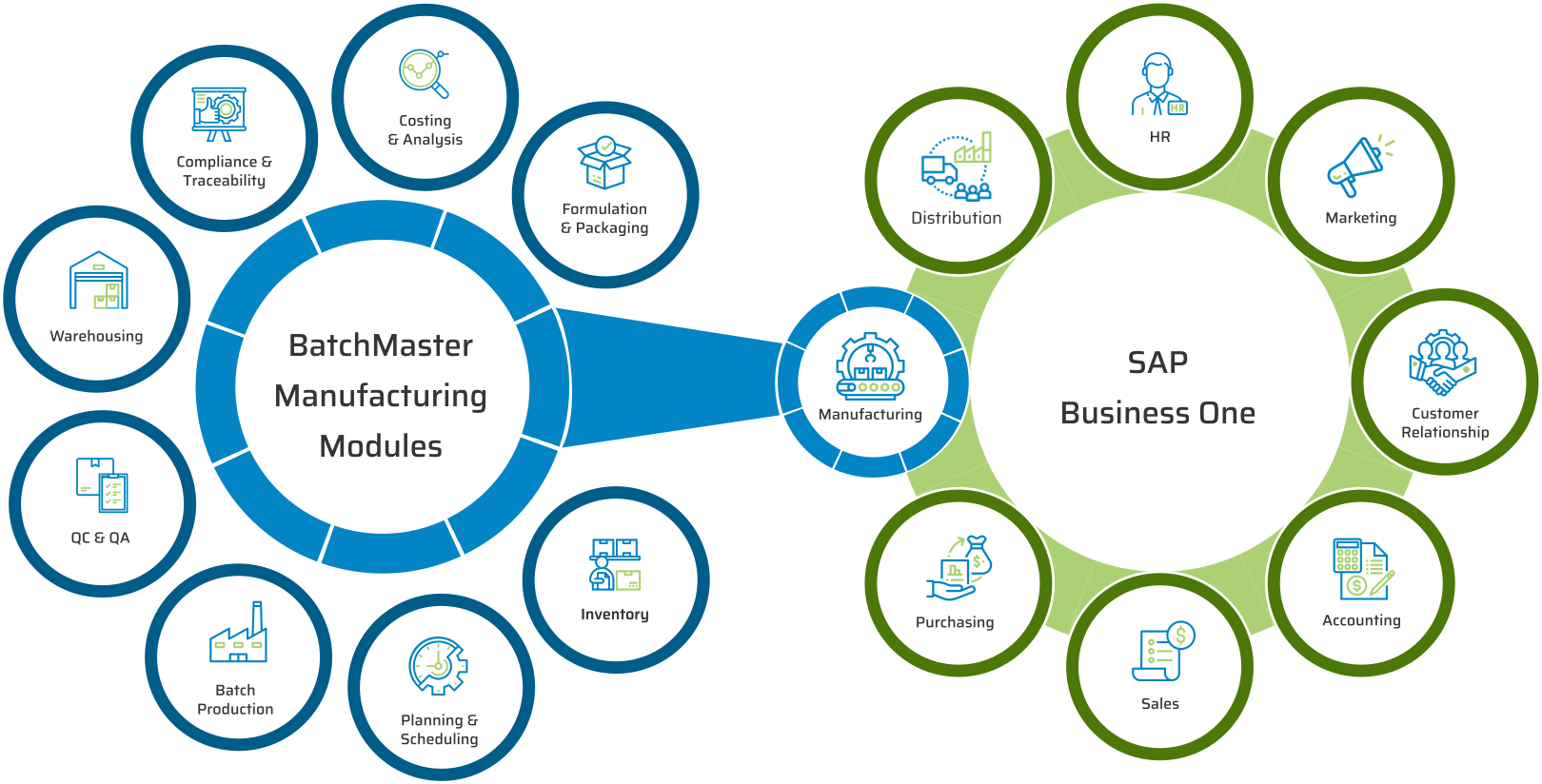
BatchMaster Software pharmaceutical and life sciences manufacturing modules include formulation and packaging management, costing, inventory, production, quality, planning, scheduling, lot traceability & recall, FDA & cGMP compliance, and mobile warehousing. All of these pharmaceutical and life sciences manufacturing modules are available in our add-on manufacturing application, which can be integrated to one’s existing financials, specifically QuickBooks, SAP Business One, Microsoft Dynamics 365 Business Central, Microsoft Dynamics GP, Sage 100 & 300. Our add-on pharmaceutical and life sciences application is available on premise and in the cloud.




UPGRADE TO OUR ERP FOR PHARMACEUTICALS & LIFE SCIENCES SOLUTION
BatchMaster ERP is the enterprise resource planning system designed to meet all of your critical business needs within a single, cohesive platform.
As an OEM partner of SAP. BatchMaster’s best-in-class business and industry capabilities for manufacturing have been natively embedded into SAP Business One, SAP’s market-leading and future-proof platform for small and midsize enterprises.
BatchMaster Software implements and supports our ERP application on our customers’ local server and in the cloud without the use of any third party consultants.
KEY MODULES FOUND IN OUR PHARMACEUTICAL & LIFE SCIENCES ERP
KEY BENEFITS OF OUR PHARMACEUTICAL & LIFE SCIENCES MANUFACTURING SOFTWARE
1. Accelerate Product Development
- Track progress of samples
- Allow multiple developers to work on separate formula and packaging specs
- Dynamically adjust formulas to meet physical and cost target values
- Perform side by side comparison of specs
- Initiate multi-level approval workflows
2. Quickly Scale Up Production
- Dynamically adjust specs and yields based upon available inventory
- Reserve inventory for customer orders
- Auto link and schedule batch jobs
- Manage equipment and resource availability and capacities
- Auto generate lot numbers for intermediates and finished goods
- Execute production related tasks via mobile devices
- Automatically backflush inventory
3. Ensure Quality
- Establish QC and QA inspection, checklist, and special instruction libraries
- Mandate task acknowledgements and quality data collection
- Auto disposition any sub standard inventory
- Generate customized COA reports
- Manage deviation and non conformance situations
4. Control Costs
- Define fixed, tiered and variable labor costs within specifications
- Identify costs of consumables
- Run what if cost analysis
- Dynamically adjust recipes to meet cost target values
- Consolidate demand using MRP and MPS for discount bulk purchases
- Optimize resources utilization based upon MRP and MPS planning
- Analyze expected vs actuals
5. Ensure Compliance
- Dynamically adjust recipes to meet label claims
- Generate FDA approved nutritional fact panels
- Generate bidirectional lot traceability reports
- Maintain full version control and audit history
- Auto assign lot numbers during receiving and production
- Track the variable characteristics of inventory
- View simultaneous dual units of measure
- Select the right inventory based upon expiry dates, certifications and status
- Reserve inventory for batch jobs
- Optimize inventory levels using automated planning and procurement
- Determine planning horizons and calendars
- Consider vendor delivery, order forecasts and planned production
- Prioritize customer orders
- Consolidate demand
- Link and schedule related batch order and runs
- Generate synchronized purchase orders
- Inbound receiving and putaway tasks
- Batch production functions and WIP inventory movements
- QC tests and special instruction tasks
- Outbound pick, pack and ship tasks
- Inventory adjustments, cycle counts and warehouse transfers
- Access real-time, accurate actionable data
- Employ drill down, role-based dashboards with graphical components
- Get quick data access using “favorite” shortcuts
- Customize industry inquiries and reports, as well as run ad-hoc queries
CONTACT US / REQUEST A DEMO
DOCUMENTS

Pharma Brochure
A 6 page brochure addressing the challenges and requirements of pharmaceutical manufacturers, and the features and benefits of BatchMaster ERP for Pharmaceuticals.

Functional Checklist
A 2 page data sheet describing key functions used in formulation, production, quality and other processes that are critical in pharmaceutical production.
VIDEOS

Speed Product Development

Streamline Production

Comprehensive QC & QA
Discover the quality control & quality assurance software features that can ensure the continuous flow of high quality products as you scale up production.

Lot Traceability & Recall
See how our lot management capabilities enable you to expedite traceability and recall activities
WHAT OUR CUSTOMERS SAY