Purpose-Built ERP Software for Nutraceutical Manufacturing
BatchMaster Nutraceutical Edition helps nutraceutical manufacturers gain control over inventory, manufacturing, traceability, quality, and compliance — from formulation to finished product.

-
Manage customer-specific specs, formulas, and docs with full
traceability and IP protection -
Speed up product launches with integrated costing
and approval workflows - Ensure label claim accuracy with potency-based formulation
- Digitally control every step of manufacturing and costing
-
Maintain product quality with real-time QC, automated SOPs,
and CAPA workflows - Stay audit-ready with built-in compliance, COAs, and digital batch records
- Reduce waste with inventory control based on shelf life and potency
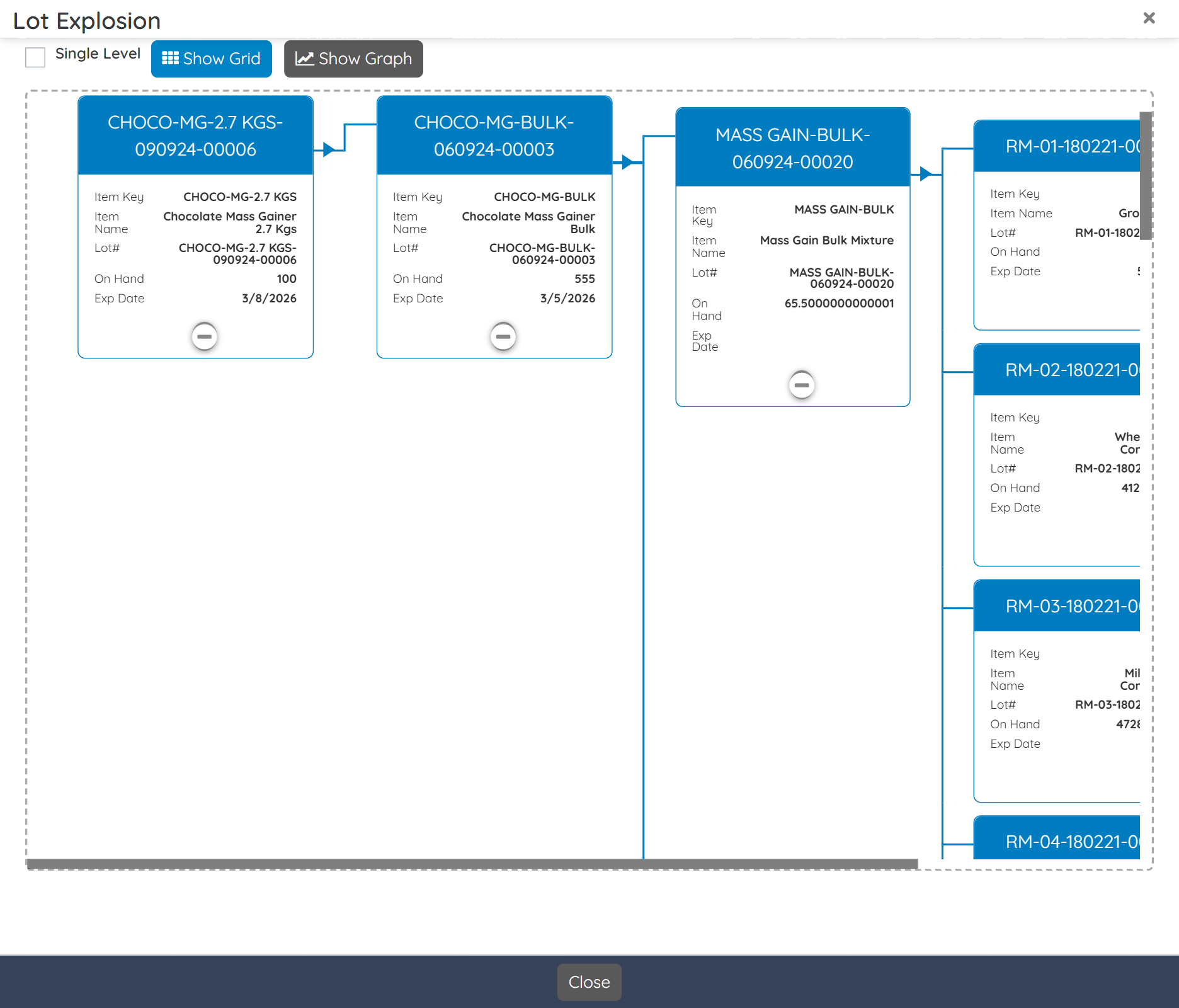
- Handle recalls fast with full batch and lot bi-directional traceability
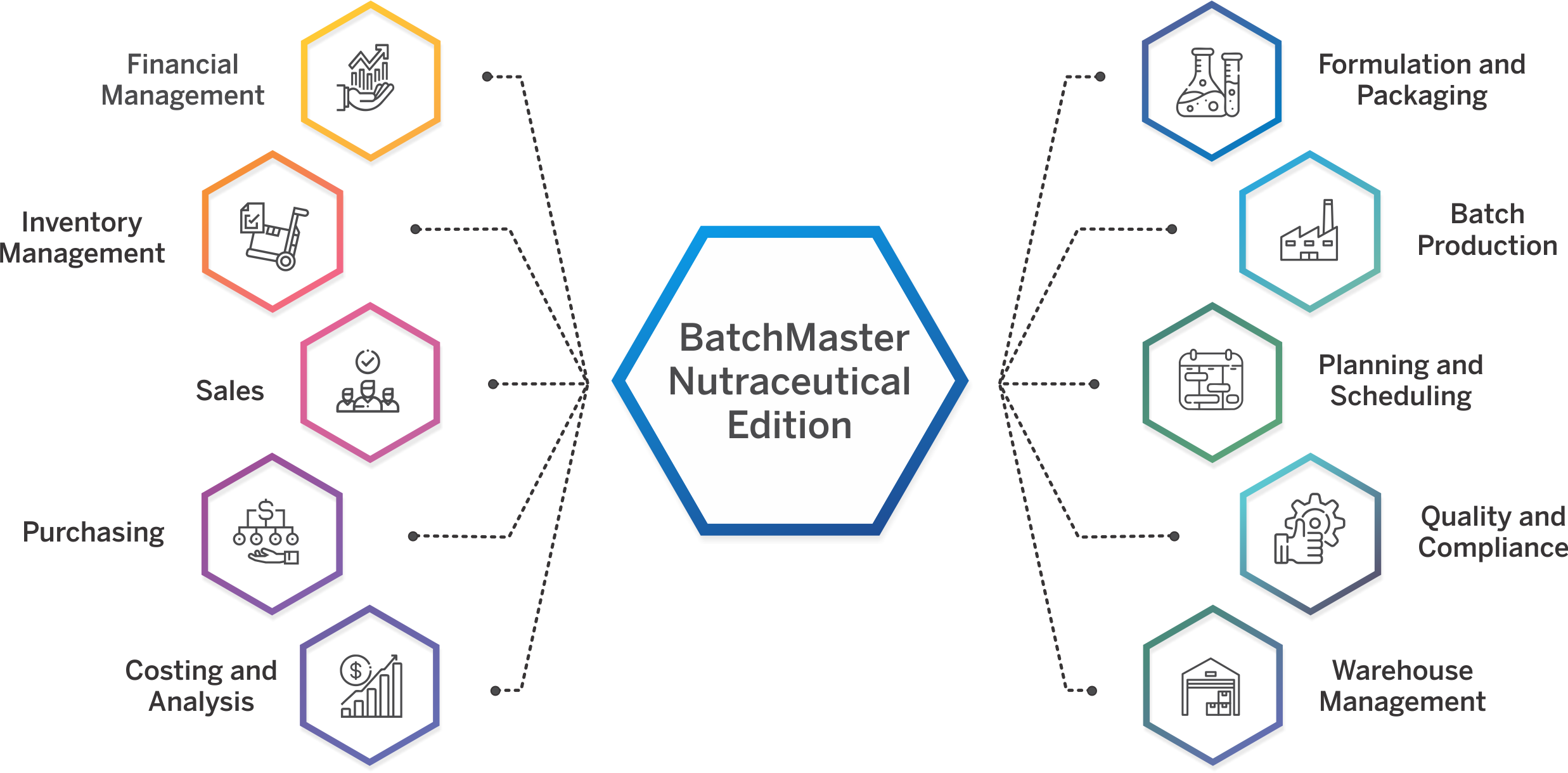
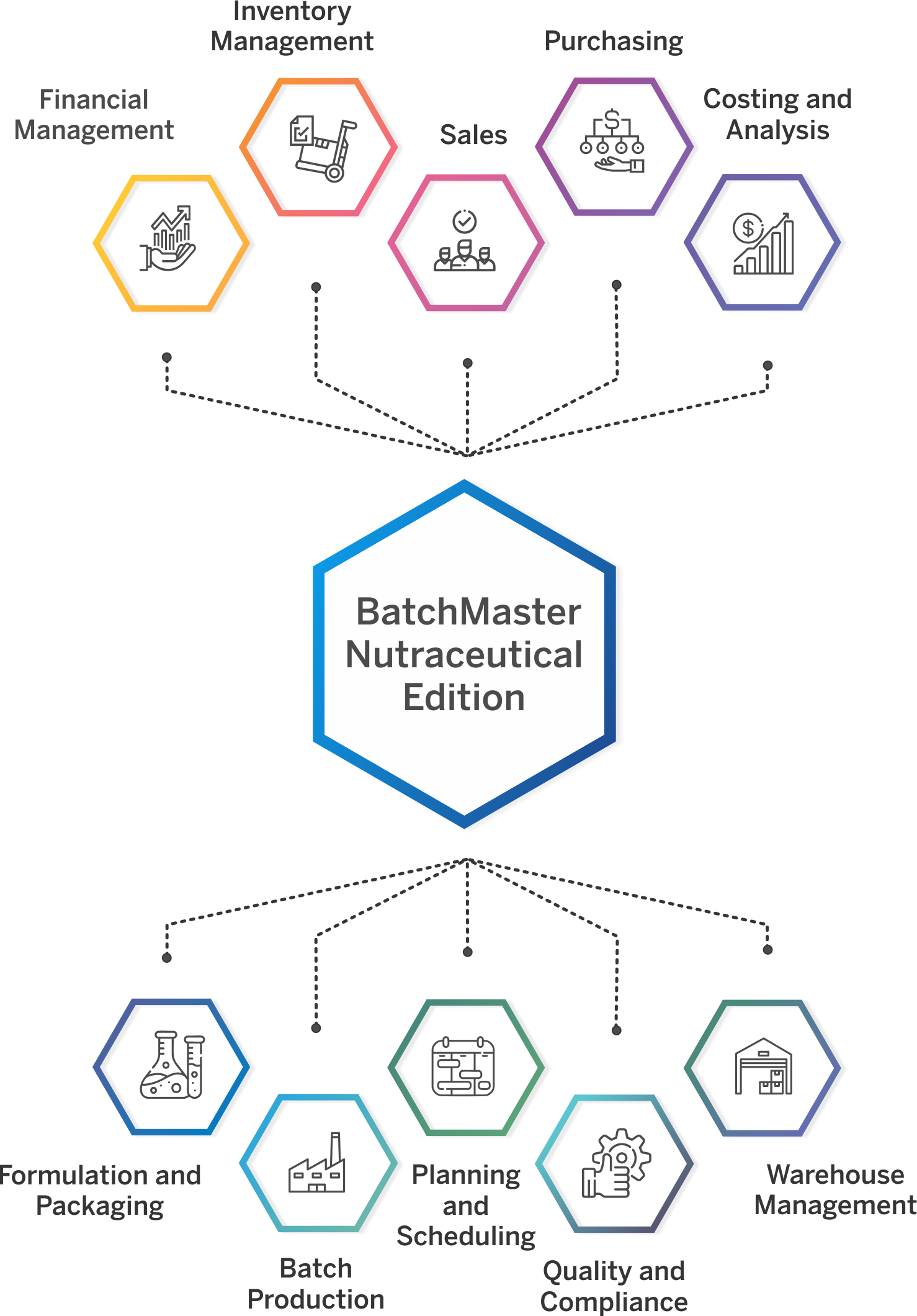
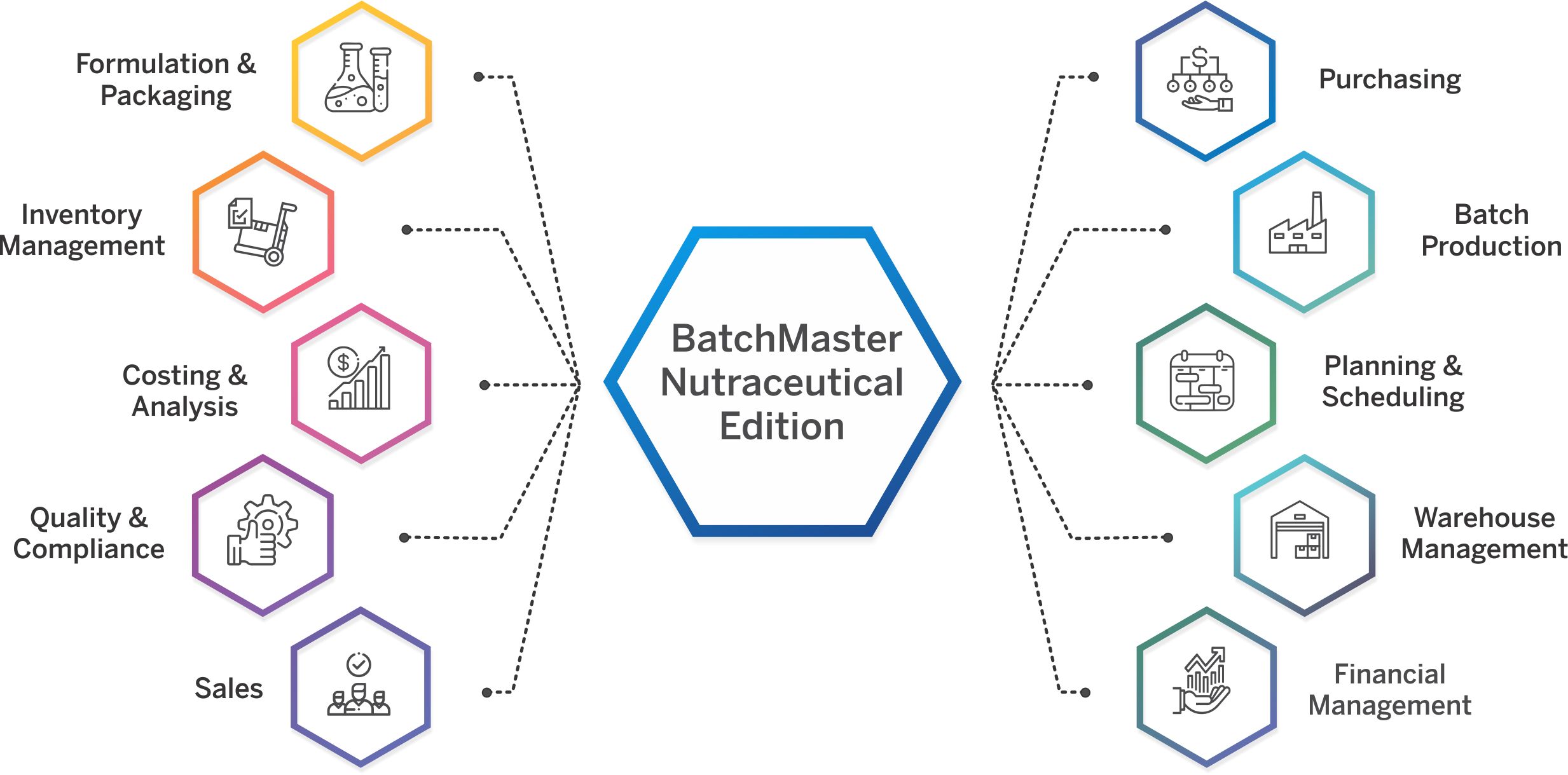
Nutraceutical ERP That Meets Your Industry Needs
Trusted by Leading Nutraceutical Manufacturers
DRIVING SUCCESS ACROSS ALL NUTRACEUTICAL SEGMENTS

Vitamins and Dietary Supplements
Vitamins, Minerals, Antioxidants and Anti-Aging, Omega-3 and Essential Fatty Acids, Amino Acids, Specialty Supplements, Chewables and Gummies

Functional Foods and Beverages
Functional Foods, Functional Beverages, Probiotics and Gut Health, Dairy Products, Liquid Shots, Specialty Juices, Snacks with Functional Benefits

Sports and Specialized Nutrition
Protein Bars and Powders, Sports Drinks, Energy Drinks for Performance, Amino Acid Supplements, Pre- and Post-Workout Nutrition, Weight Management Products

Medicinal and Botanical Products
Herbal Supplements, Plant-Based and Vegan Nutrition, Botanical Extracts, Natural Functional Foods, Ayurvedic and Traditional Medicine Products, Organic Nutrition, Medicinal Mushrooms
Specialized Capabilities for Nutraceutical Operations
Control every stage of product development, from managing formulas, packaging specs, and label claims to pre-approvals and SOPs. Centralize data and approvals to reduce delays and bring new products to market faster.
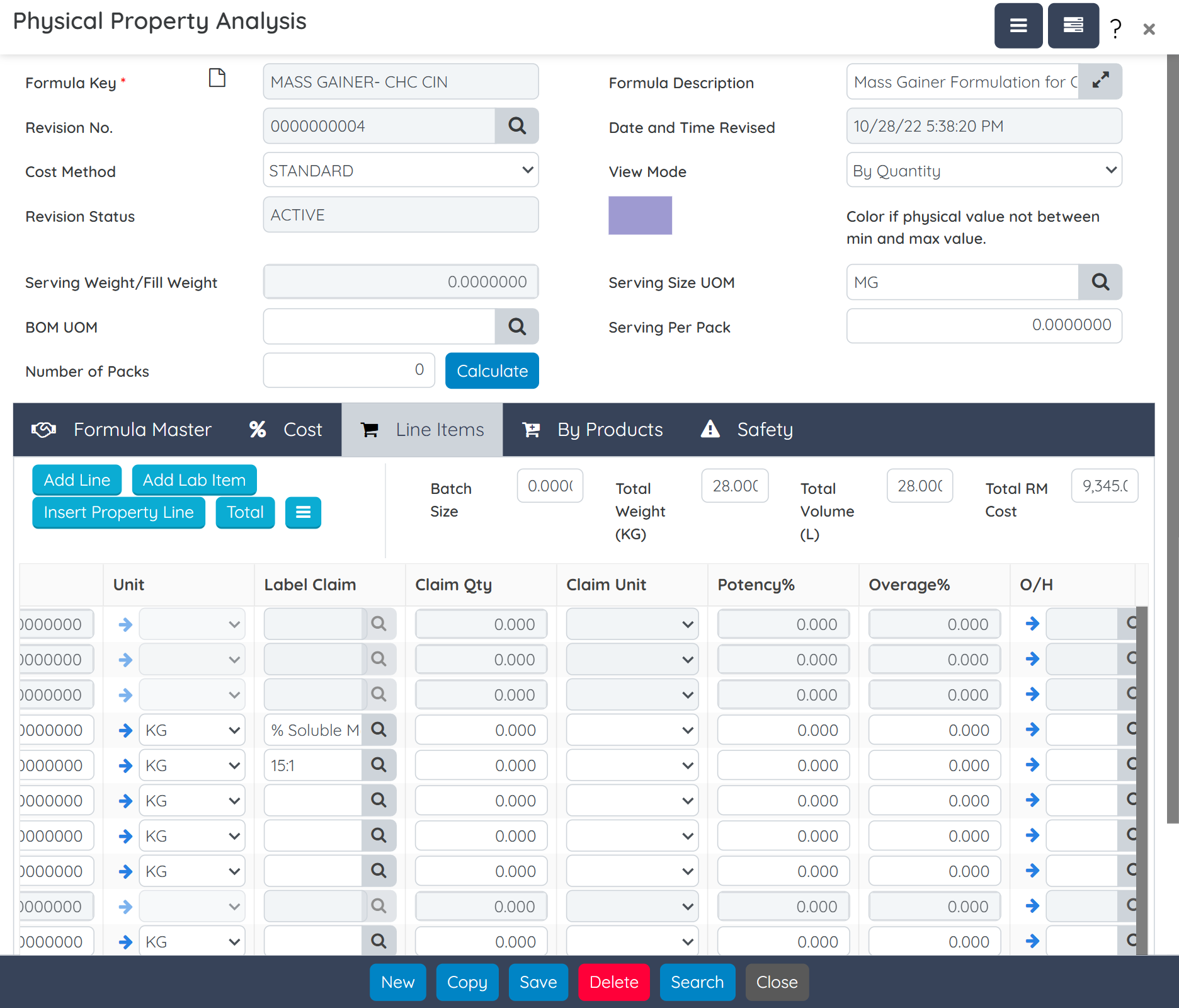
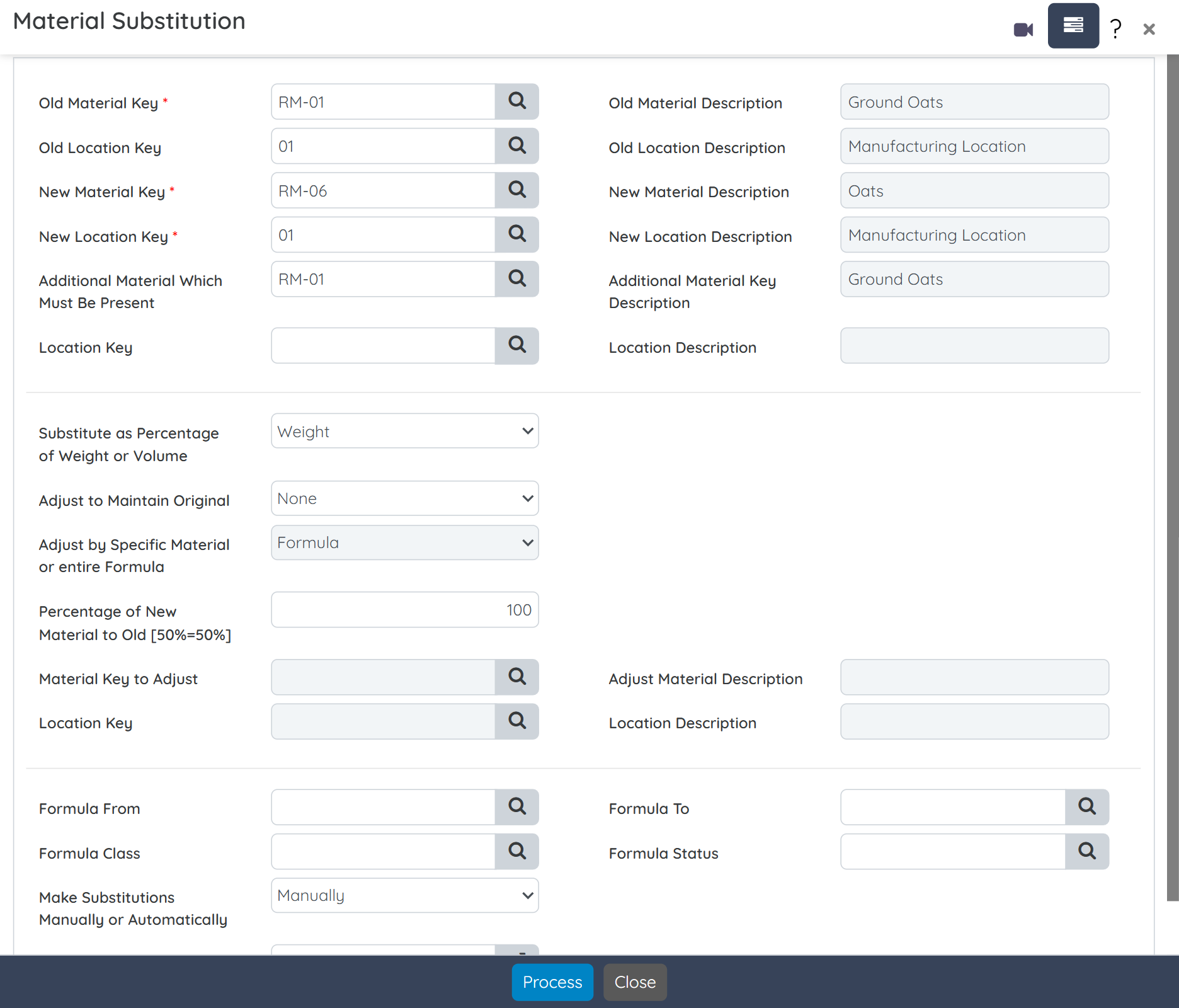
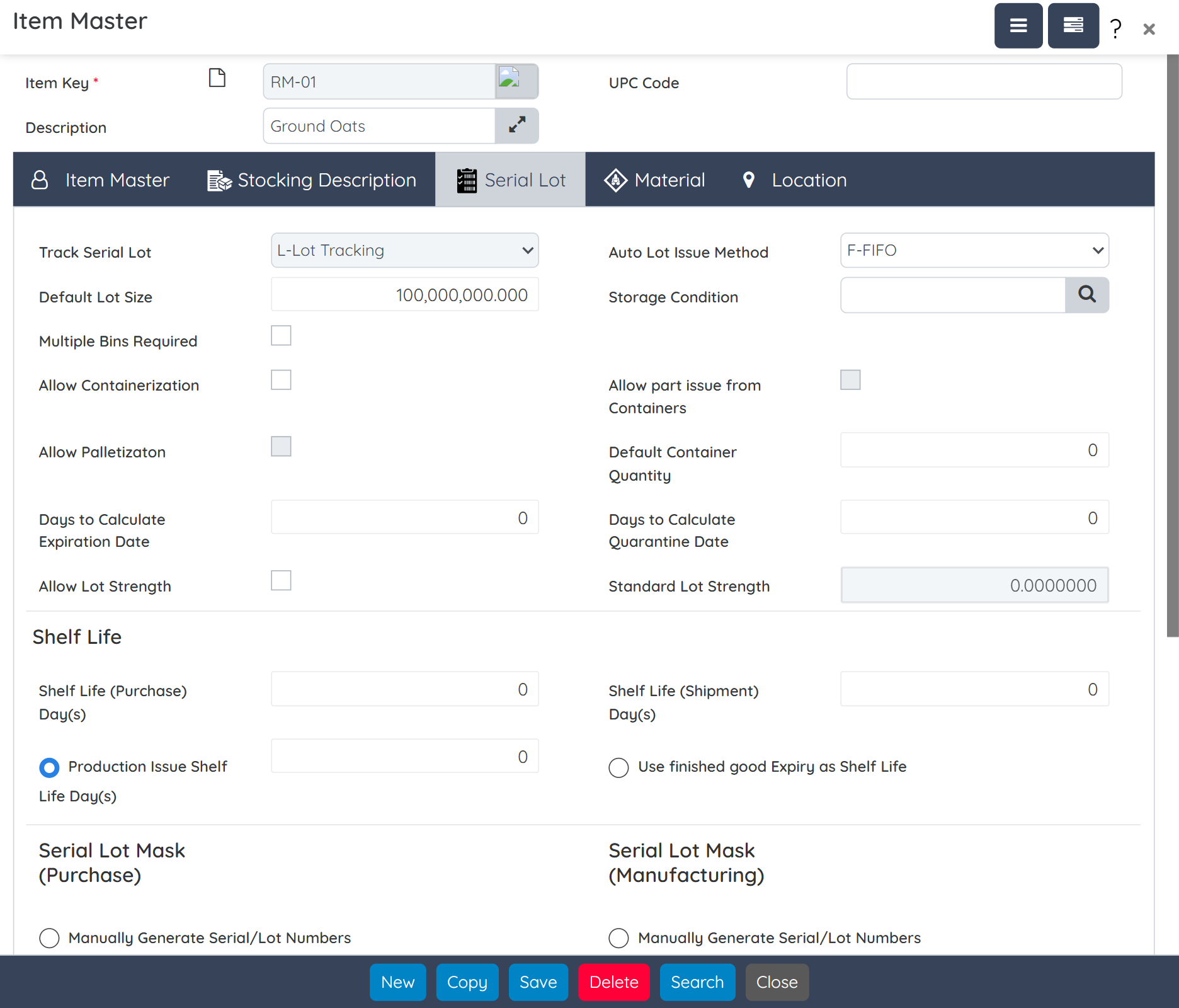
Create precise formulas that meet label claim requirements while accounting for potency variability in natural ingredients. Define dosage forms, manage multiple packaging specs, and auto-generate supplement facts, all from a single screen. Built-in tools also allow raw material substitution with full impact tracking. Intellectual property is protected with role-based security and alias naming for sensitive formulations.
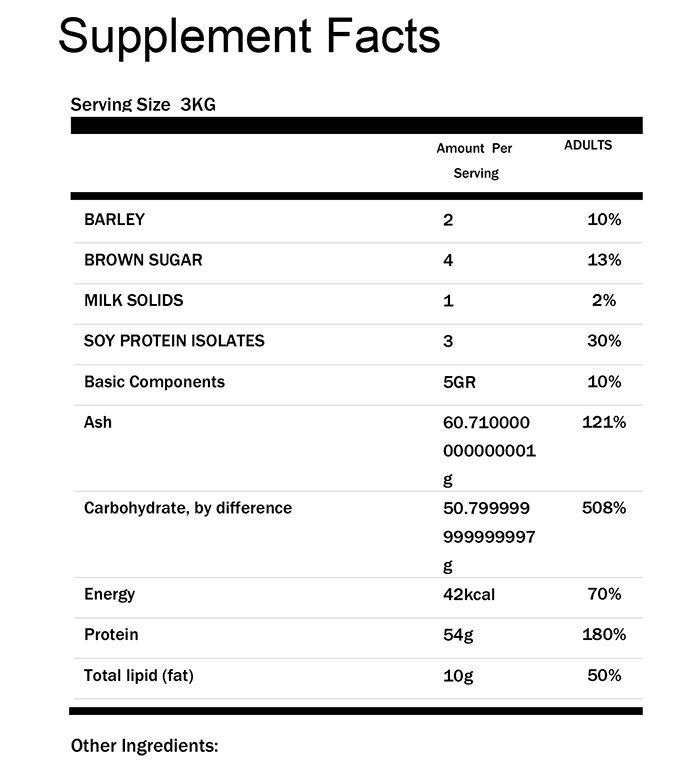
Auto-generate compliant supplement facts panels, ingredient declarations, and allergen-safe labels directly from your formulation data. Eliminate manual formatting and support multiple packaging configurations with built-in templates that meet FDA requirements.
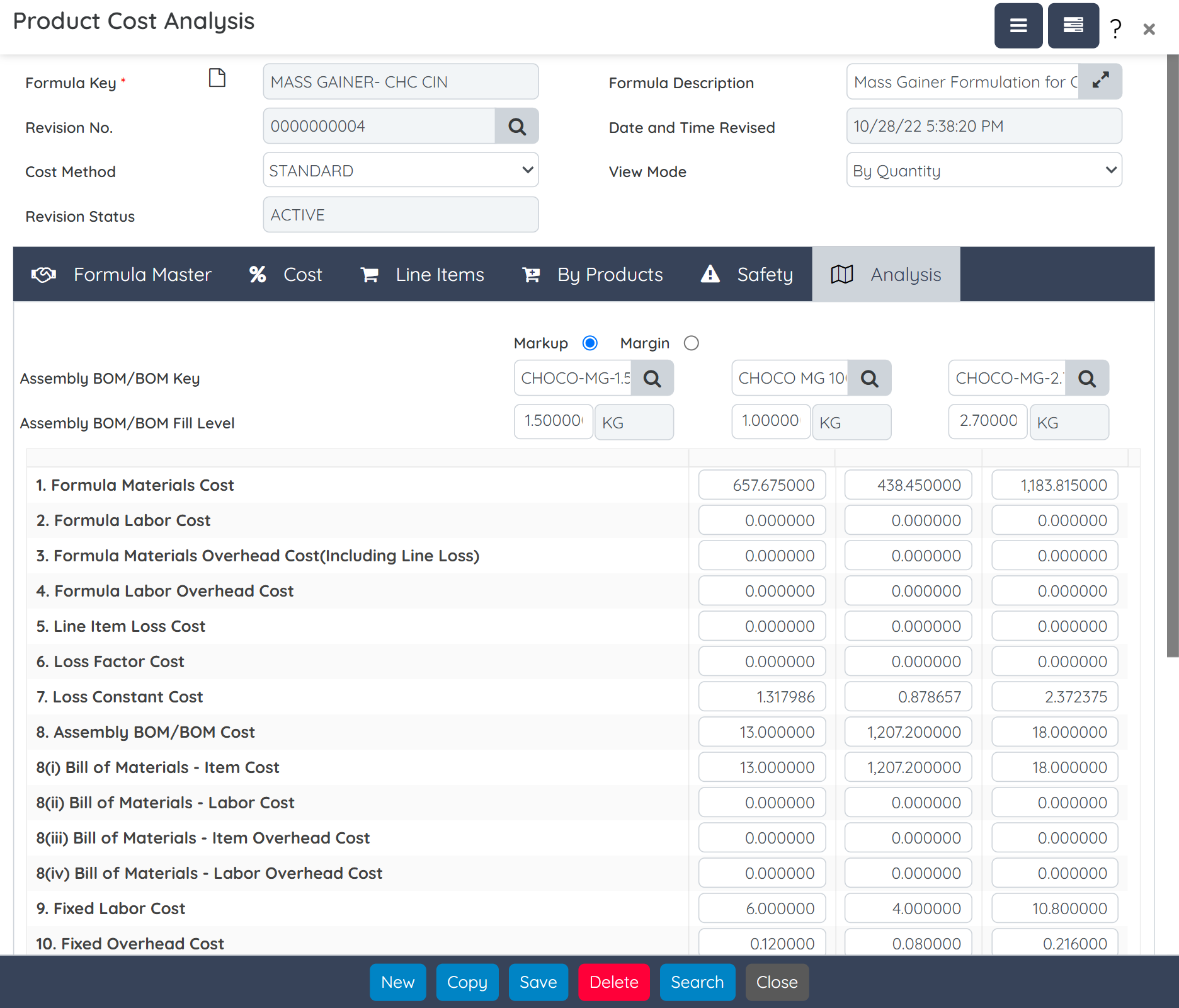
Perform “what-if” costing analysis during product development to evaluate ingredient, labor, and packaging costs. Run margin checks and integrate with CRM to support quick quote generation and cost-based decision-making.
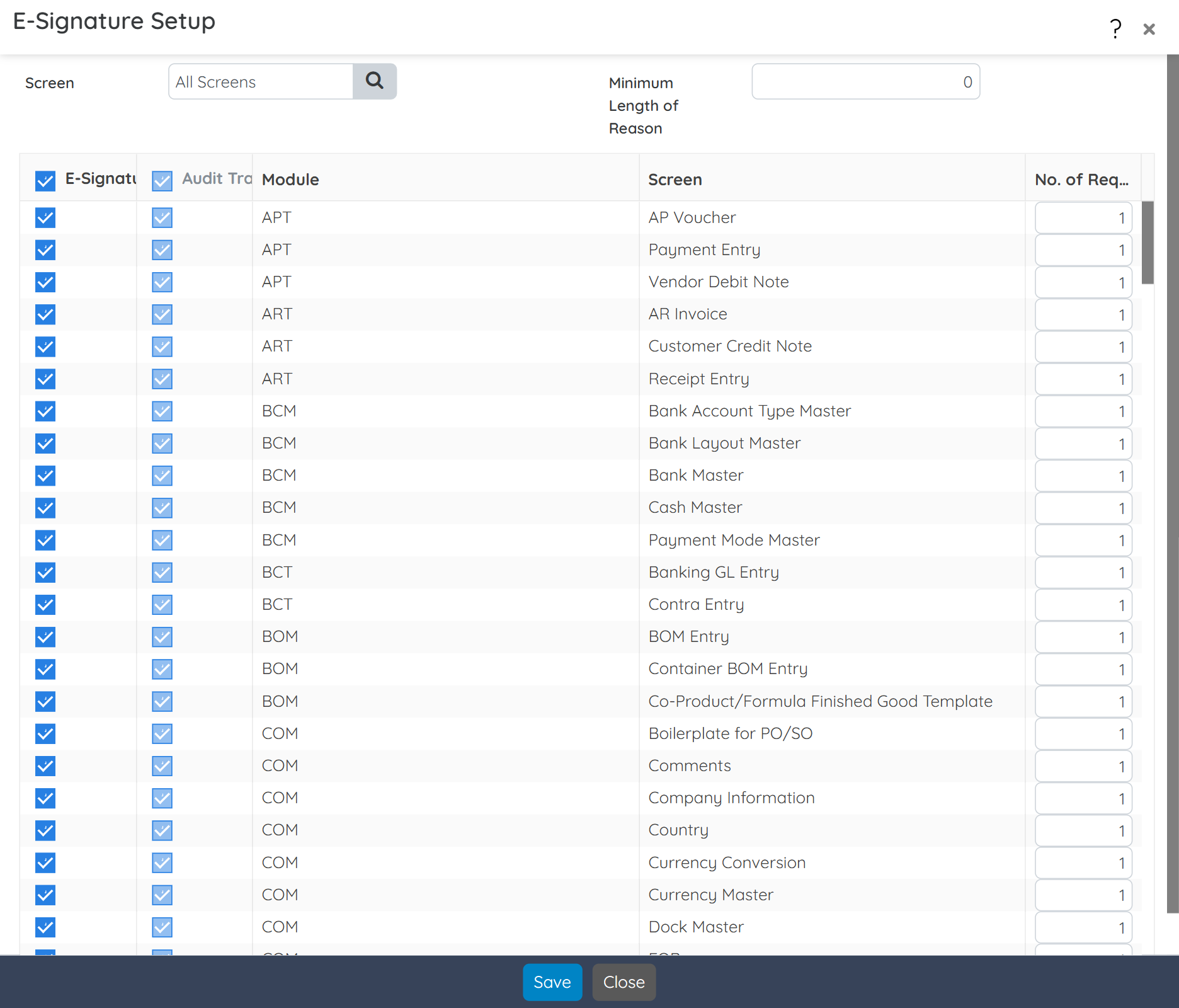
Ensure full cGMP compliance with built-in validation templates, SOP enforcement, and vendor qualification tools. Track and document every production step, deviation, and material movement with audit-ready details. BatchMaster also supports 3rd-party validation efforts during ERP implementation, helping accelerate your path to regulatory readiness.
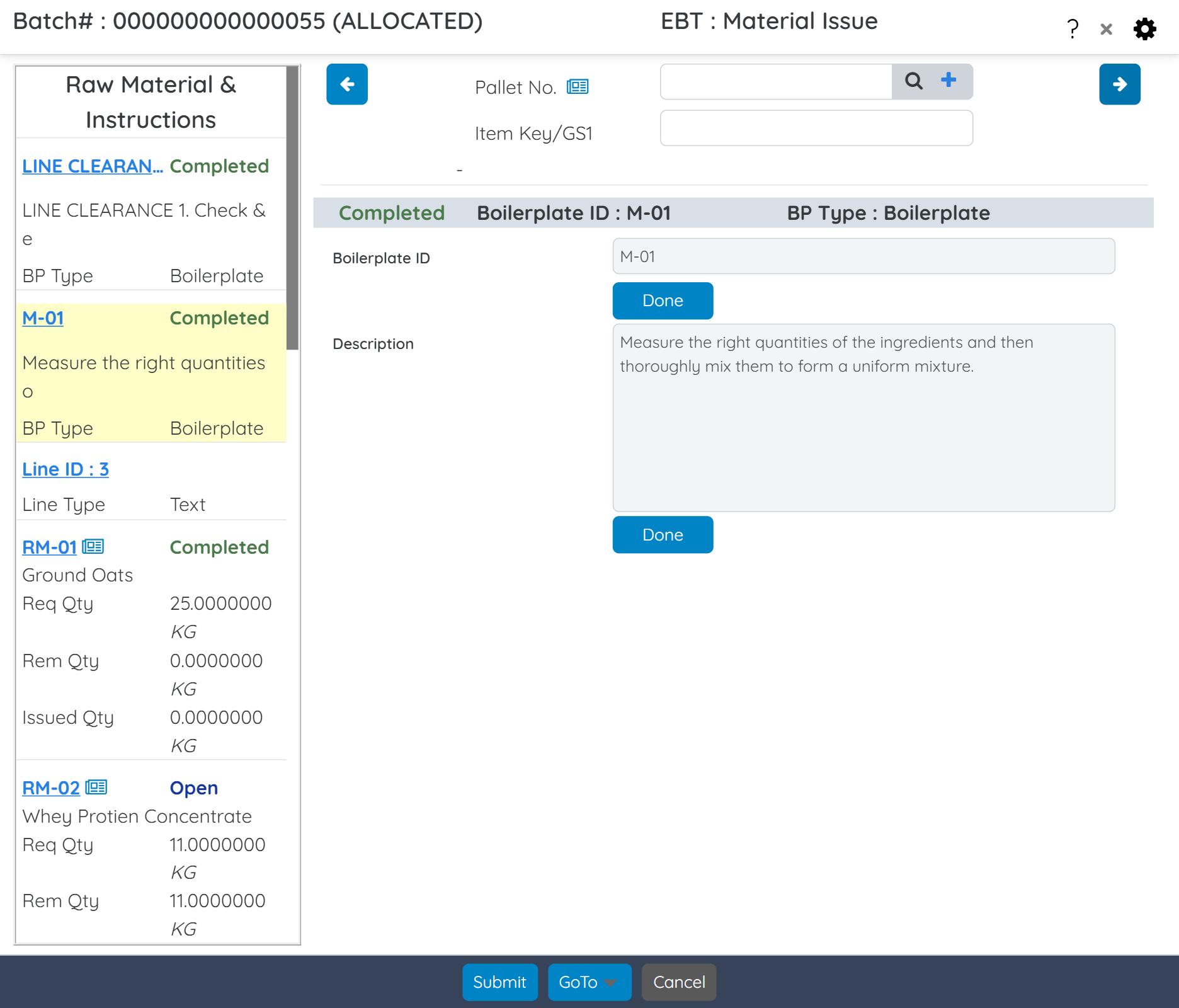
Support digital batch execution with Electronic Batch Tickets (EBT), which eliminates paperwork, captures execution data in real time, and routes digital approvals for faster, error-free production. Digitally capture every batch activity—ingredients, procedures, signatures, and validations—in a centralized, secure system. Eliminate paper logs and maintain 21 CFR Part 11-compliant Master Batch Records with complete traceability, audit trails, and real-time production visibility.
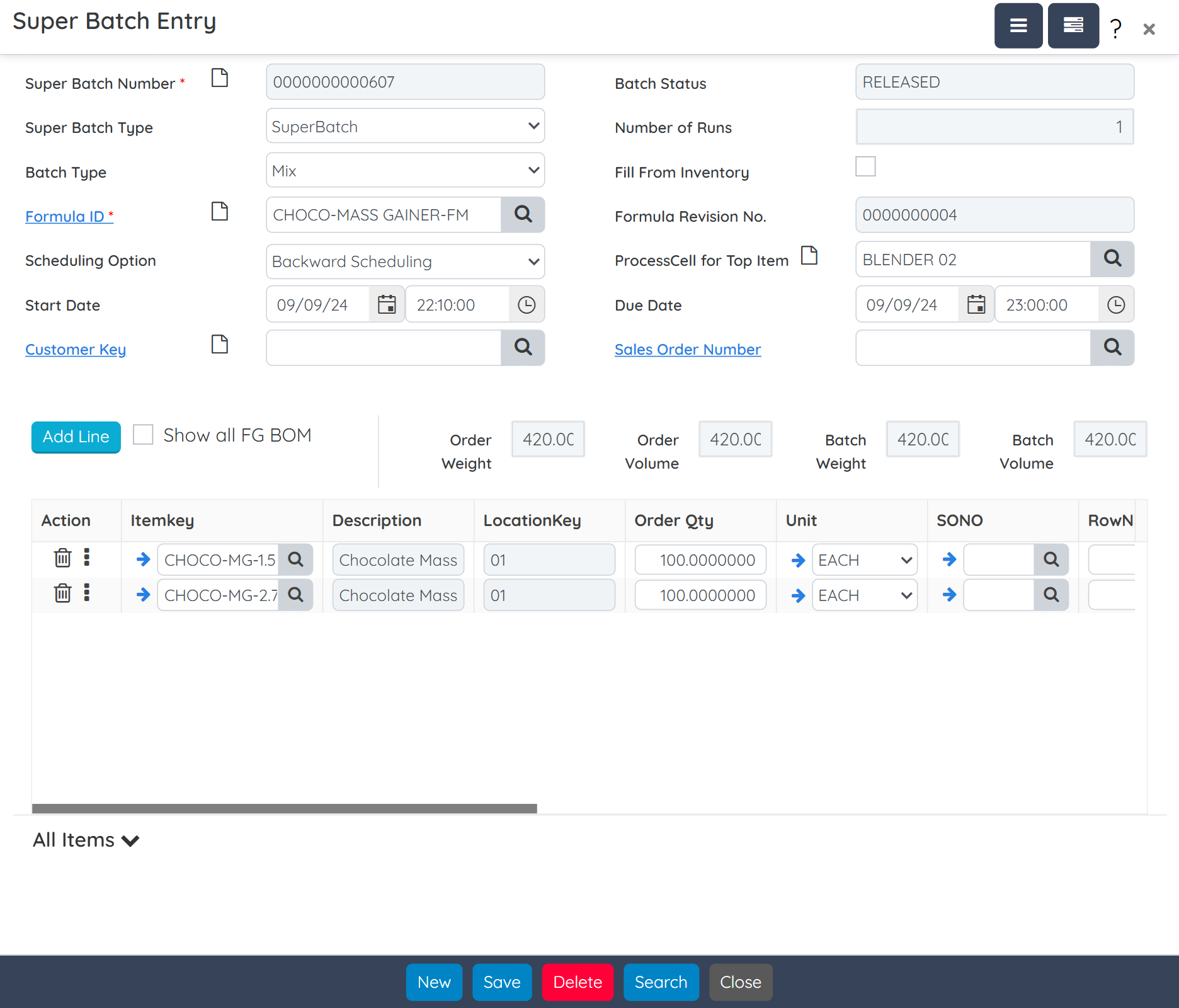
Handle campaign, super batch, and sub-batch scenarios across multiple lines or facilities. Digitally capture SOPs, batch weights, and shop-floor activity using integrated weigh sheets. Production teams gain real-time visibility into job progress, ingredient consumption, and resource utilization, ensuring consistent execution and complete traceability.
Run multi-level quality checks from raw materials to finished goods. Automate COA generation, track stability tests, manage non-conformances, and execute CAPA workflows. Include training management, inspection plans, SOP control, and audit trail maintenance for optimal quality control. Ensure quality assurance at every step, for example defining and enforcing item-specific acceptance criteria for raw materials and finished goods to ensure consistent product quality before release.
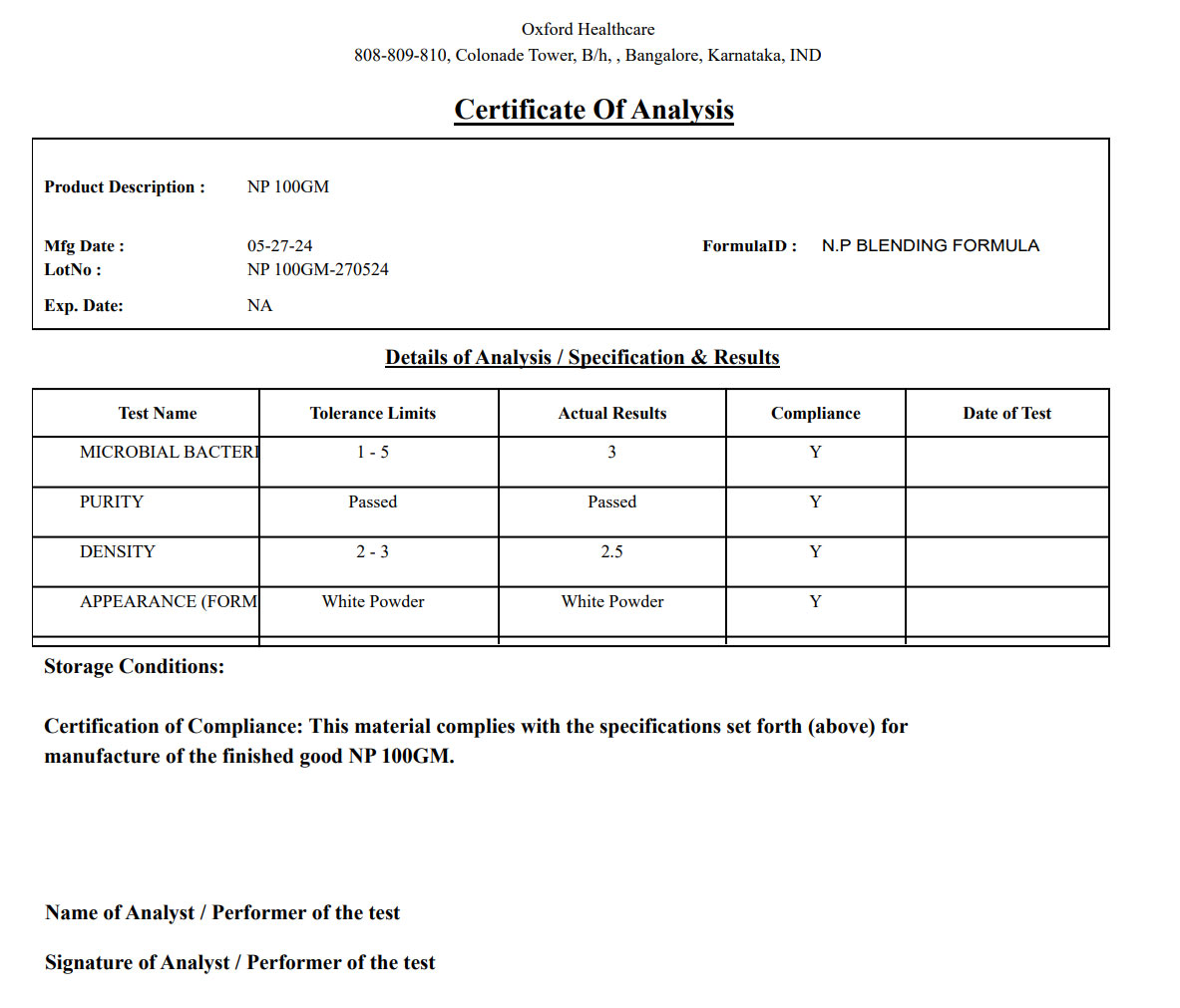
Meet FDA, cGMP, FSMA, and 21 CFR Part 111/11 requirements with built-in tools for managing deviations, label claims, SOPs, and electronic batch records. Full support for electronic signatures, version control, and audit trails helps you stay compliant across every regulated step of production.
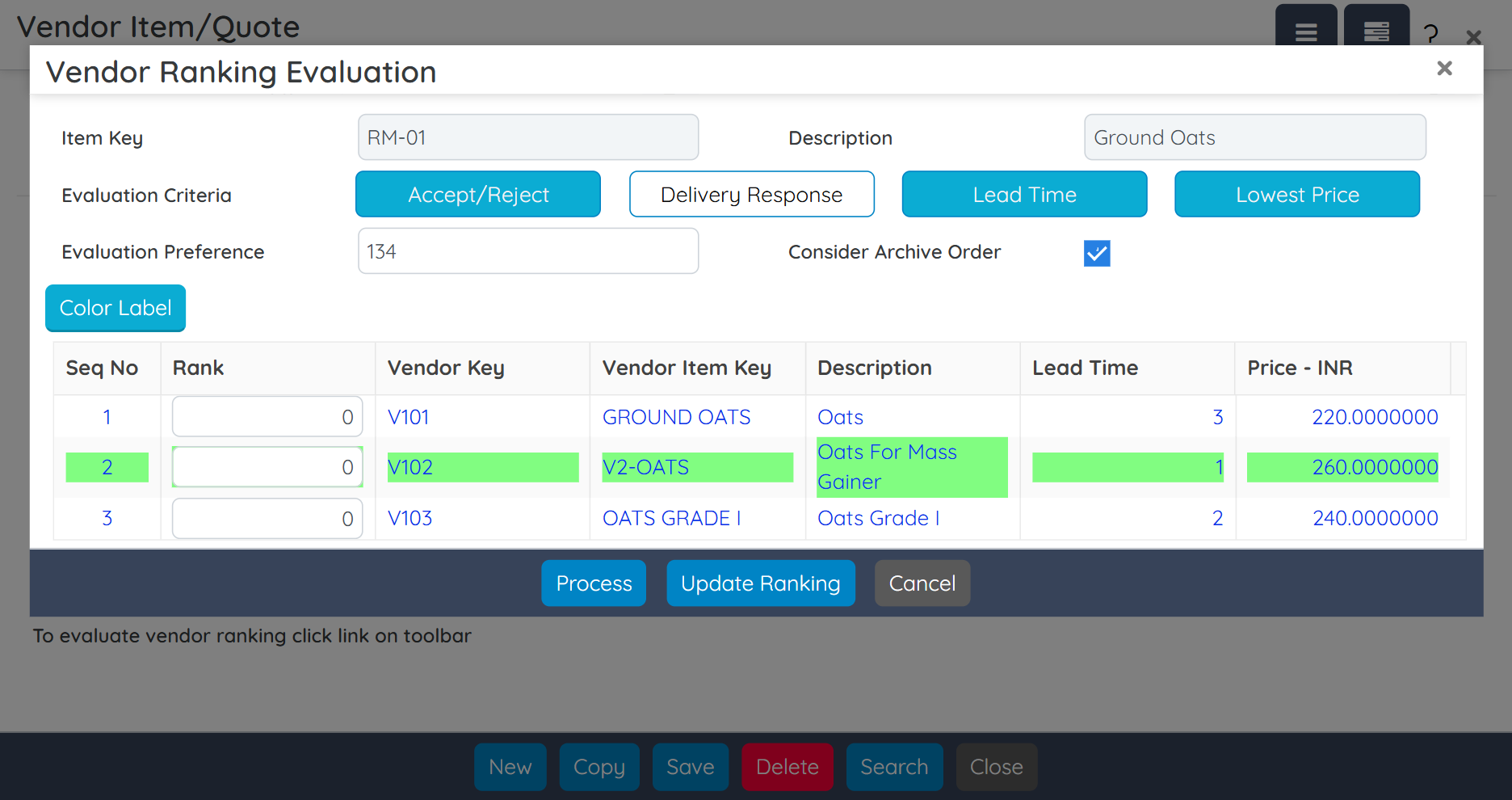
Track inventory by potency, shelf life, FEFO/FIFO, and lot classification across multiple warehouses. Real-time integration with barcode scanners, RF devices, mobile WMS, and electronic scales ensures accuracy. Advanced MRP/MPS tools align procurement and production with demand and expiration data.
Gain complete bi-directional traceability from raw materials to finished goods. Instantly recall products with full visibility into lots, blends, and packaging components. Maintain audit-ready records to comply with internal, customer, and regulatory traceability demands.
Automatically track allergens and generate accurate ingredient lists by weight or volume. Monitor and manage certifications like Organic and Non-GMO with expiration alerts to ensure continued compliance in labeling and formulation.
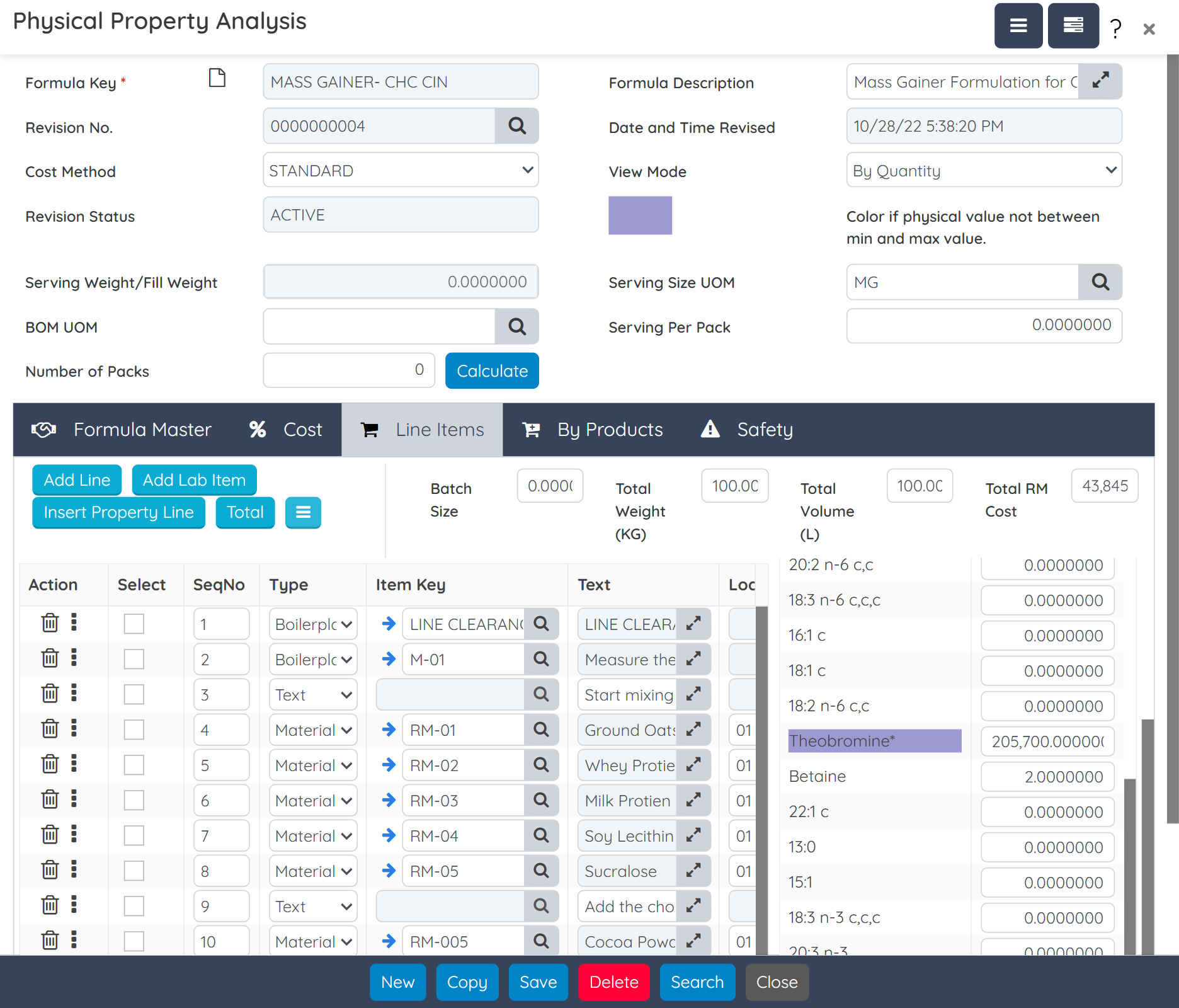
Handle customer-supplied materials, client-specific specs, and multi-version documentation with ease. Provide private-label and white-label clients with 24/7 portal access to monitor orders, shipments, labels, and documents. Include support for IP protection, segregated inventory, and subcontracted production flows. BatchMaster also supports own-brand nutraceutical manufacturers selling direct-to-consumer with seamless Shopify integration that connects e-commerce storefronts to inventory, order fulfillment, and production.













Hear from Leading Nutraceutical Manufacturers
Integrates with Leading Financial Systems





Why Nutraceutical Manufacturers Choose BatchMaster
BatchMaster Nutraceutical Edition is built to help nutraceutical manufacturers grow, stay compliant, and scale efficiently. Our fully cloud ERP delivers the depth, expertise, and intelligence that formula-based manufacturing demands. With implementation, support, and services handled entirely by our in-house team, you gain a partner who deeply understands your nutraceutical operations.
Why Nutraceutical Manufacturers Choose BatchMaster
BatchMaster Nutraceutical Edition is built to help nutraceutical manufacturers grow, stay compliant, and scale efficiently. Our fully cloud ERP delivers the depth, expertise, and intelligence that formula-based manufacturing demands. With implementation, support, and services handled entirely by our in-house team, you gain a partner who deeply understands your nutraceutical operations.


Experience the ERP Built for Nutraceutical Success
Built to solve the toughest challenges in nutraceutical manufacturing, from managing potency variability to meeting strict regulatory demands.
BatchMaster Nutraceutical Edition Resources
BatchMaster Nutraceutical Edition: FAQs
Yes. Whether you’re an early-stage startup, a growing mid-sized business, or a well-established nutraceutical brand, BatchMaster is designed to scale with you. We address the unique needs of small and medium businesses with industry-specific functionality that supports fast growth. Additionally, our solution meets the complex demands of large, established nutraceutical enterprises through advanced capabilities, deeper configurability, and enterprise-level scalability.
Yes. The system supports tablets, capsules, powders, soft gels, liquids, and more. You can define and manage different packaging and formulation versions for each product.
BatchMaster includes tools for managing 21 CFR Part 111/11, cGMP, FSMA, and other standards. Electronic batch records, audit trails, COA generation, and traceability features help you stay audit-ready at all times.
No. BatchMaster supports a wide range of nutraceutical manufacturing models—including own-brand, private-label, and contract manufacturing. It offers robust tools to manage customer-specific formulations, documentation, and inventory, along with features like role-based security and alias naming to protect intellectual property across all business types.
Implementation timelines vary depending on the complexity of your operations, but most nutraceutical customers go live within a few months. Our in-house team manages the entire process and provides industry-specific templates to accelerate setup.
Yes. BatchMaster integrates seamlessly with QuickBooks, Microsoft Dynamics Business Central and GP, and Sage 100/300. You can also use BatchMaster’s built-in financials for a fully unified system.
You’ll have access to our in-house support team with expertise in nutraceutical manufacturing. We offer live assistance, training, and a customer portal for ongoing help and updates.